On 5 July 2023, Mr. Xu Jinghe, Deputy Commissioner of China’s National Medical Products Administration (NMPA), during the press conference “Talk by Authorities” illustrated the efforts that China has made, over the past five years, in optimizing and improving the medical device standards system. Currently, a total of 1937 medical device standards are in force, while the degree of consistency with international standards has exceeded 90%. Around 18 standards are the newly-formulated in 2023.
Every year, NMPA issues a report on the progress of China’s medical device standards. To better help foreign stakeholders understand the progress, we summarize the key points below:
Technical committees. By the end of 2022, a total of 37 medical devices standardization technical committees had been established in China, including 13 technical committees, 13 technical subcommittees, 1 standard working group, and 10 mirroring committees (see Figure 1).
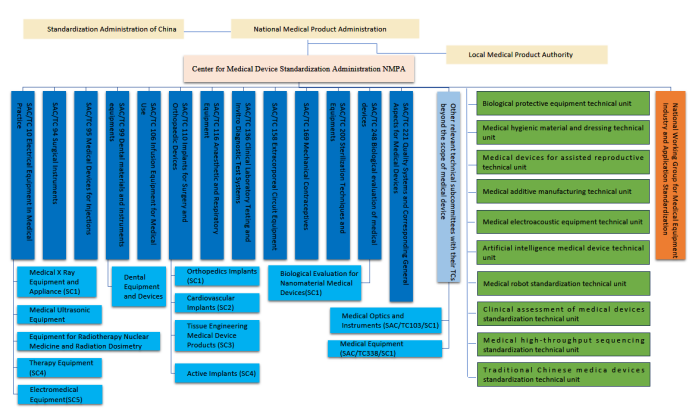
Figure 1: Technical Committees for Medical Devices Standardization in China
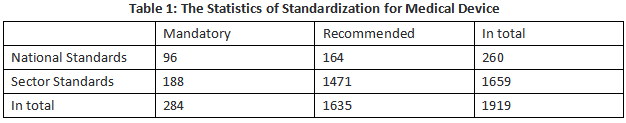
Standardization. In 2022, a total of 42 national standard projects on medical device were approved, together with 117 sector standard projects (of which are 23 mandatory, and 94 recommended). Furthermore, a total of 40 national standards and 114 sectoral standards for medical devices were issued, together with 4 newly-revised standards were issued. By the end of 2022, there were 1,919 active standards on medical devices (see Table 1).
Covered areas. The medical device standards cover various technical fields, such as electrical equipment in medical practice, surgical instruments and surgical implants. The top three areas covered are: medical laboratory equipment (14%), orthopedics and orthopedics instruments (11%), and general surgical and microsurgical instruments (11%).
International standards. Three China-led international standards were officially released. These are:
- ISO 8536-15:2022 Infusion equipment for medical use — Part 15: Light-protective infusion sets for single use
- ISO/TS 5798:2022 In vitro diagnostic test systems — Requirements and recommendations for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by nucleic acid amplification methods; and
- ISO/TS 24560-1:2022 Tissue-engineered medical products — MRI evaluation of cartilage — Part 1: Clinical evaluation of regenerative knee articular cartilage using delayed gadolimium-enhanced MRI of cartilage (dGEMRIC) and T2 mapping.
In 2022, there was a significant increase in the number of applications for the conversion of international medical device standards into Chinese standards, with a total of 117 filings – twice the number recorded in 2020. This surge in applications aims to enhance consistency with international standards.




